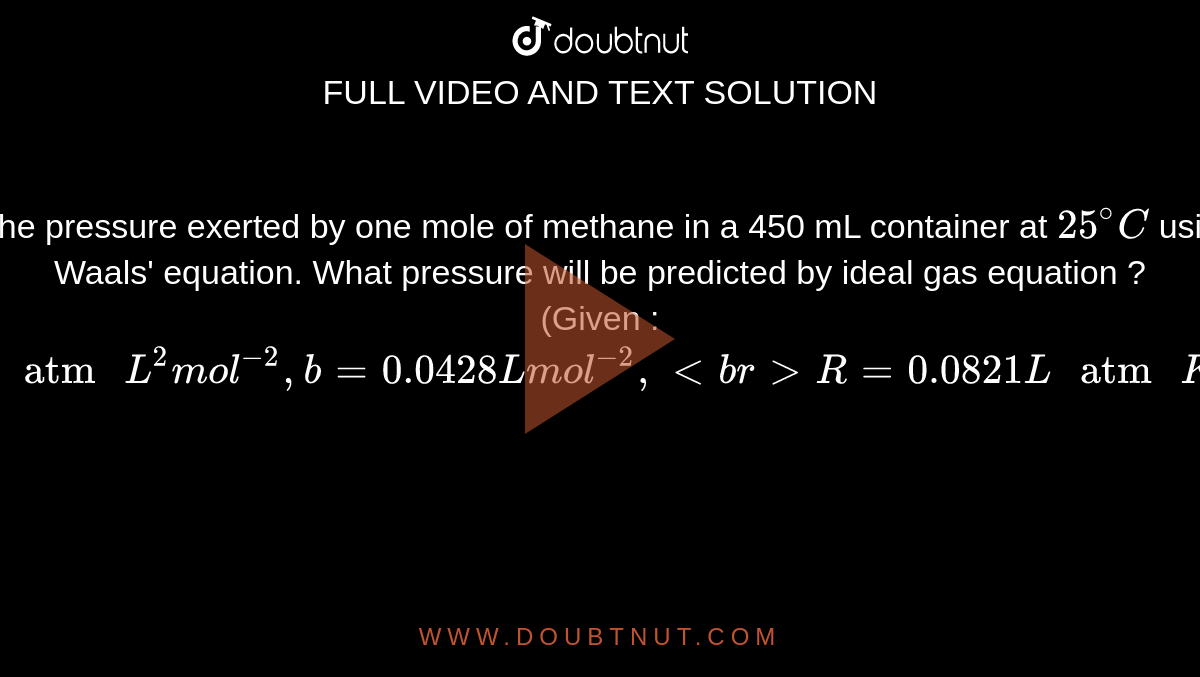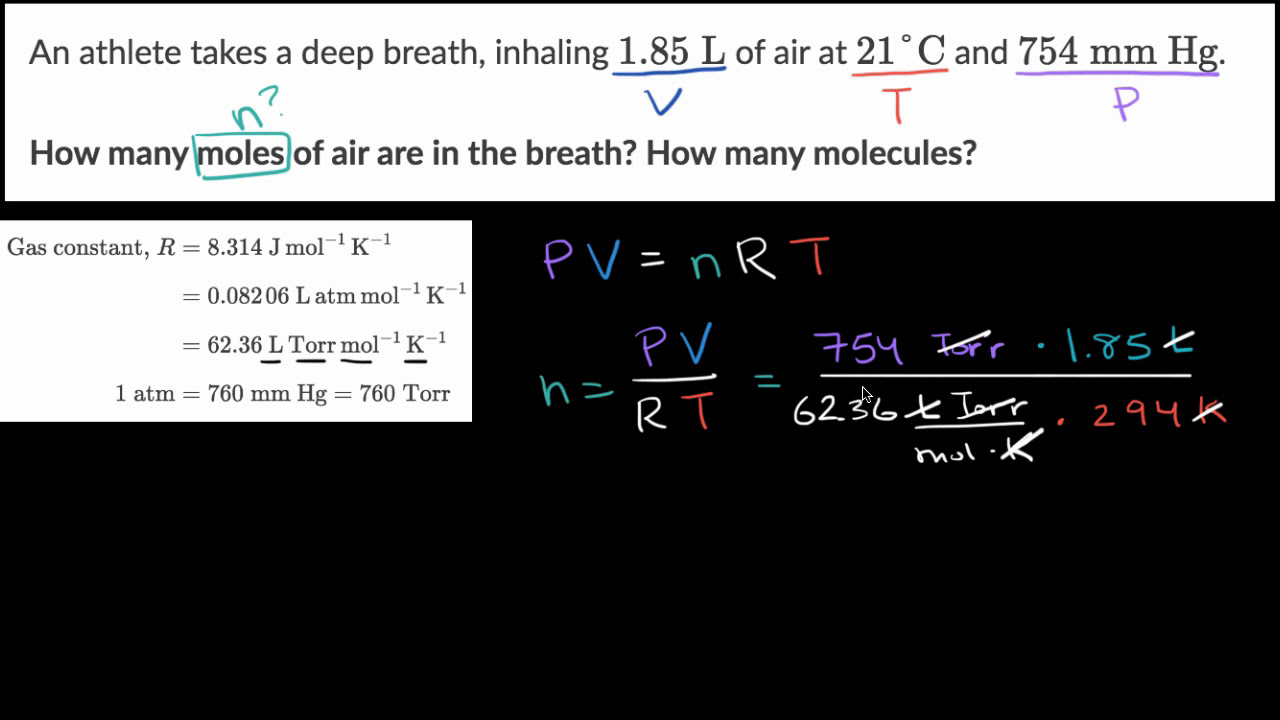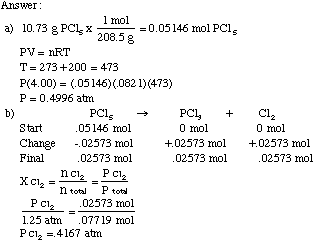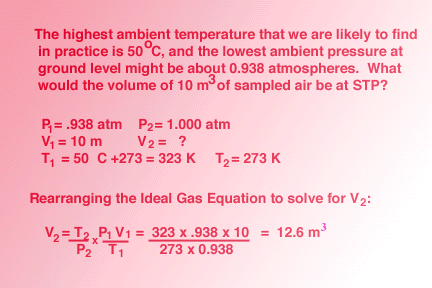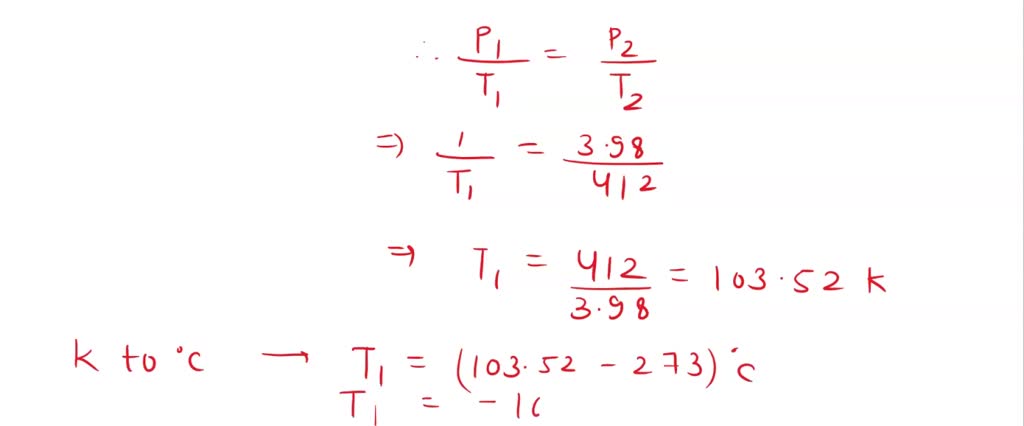
SOLVED: An ideal gas is heated at constant volume During this process the pressure increased from 1.00 atm to 3.98 atm. If the final temperature of the gas is found to be

Calculate the number of moles of gas present in the container of volume 10 L at 300 K. If the manometer containing glycerin shows 5 m difference in level as shown diagram.

An ideal gas at 0.80 atmospheres and 87°C occupies 0.450 liter. How many moles are in the sample? - YouTube

6.3: Combining the Gas Laws: The Ideal Gas Equation and the General Gas Equation - Chemistry LibreTexts
Five moles of ideal gas expand isothermally and reversibly from pressure 10 ATM to 2 ATM at 300 K. What is the largest mass which can be lifted through a height of
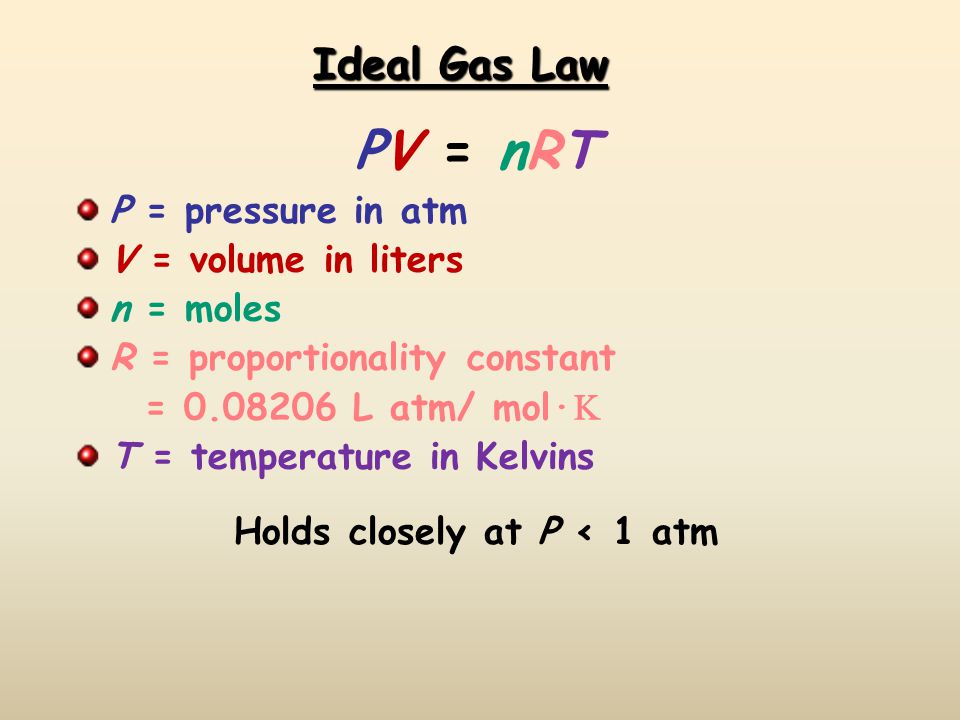
If I have 21 moles of gas held at a pressure of 3800 torr and a temperature of 627°C what is the volume of the gas? | Socratic

Calculate the temperature of 4.0 moles of a gas occupying 5 dm^3 at 3.32 bar (R = 0.083 bar dm^3 K^-1 mol^-1) .